CIMZIA Background
For the treatment of adults with moderate to severe plaque psoriasis (PSO) who are candidates for systemic therapy or phototherapy, and adults with active psoriatic arthritis (PsA)1
CIMZIA®
(certolizumab pegol)
is backed by a rich history of helping patients1,2
6 FDA‑approved indications with a demonstrated safety profile1a
PSO: Moderate to severe plaque psoriasis in adults who are
candidates for systemic therapy or phototherapy
PsA:
Active psoriatic arthritis
NR-axSpA: Active non-radiographic axial spondyloarthritis
with objective signs of inflammation
AS: Active ankylosing
spondylitis
RA: Moderately to severely active rheumatoid
arthritis
CD: Moderately to severely active Crohn’s disease
if response to conventional therapy is inadequate

More than 644,000 patient‑years of worldwide cumulative exposure across approved indications2b
YEARS
YEARS
80
aIncludes approved indications
for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis,
ankylosing spondylitis, non-radiographic axial spondyloarthritis,
and plaque psoriasis, as well as other completed and ongoing
research.
bPatient exposure
was estimated using the available sales data in rheumatoid
arthritis, Crohn’s disease, axial spondyloarthritis (including
ankylosing spondylitis and non-radiographic axial
spondyloarthritis), plaque psoriasis, and psoriatic arthritis from
Sep 01, 2007, to Feb 28, 2019, for the cumulative time interval.
The exposure of CIMZIA was calculated using the following formula:
Patient-years = ([total mg of product distributed]/[monthly
maintenance dose])/12 months in year.
cCIMZIA was first approved by the FDA in April 2008 for adults
with moderate to severe Crohn’s disease.
dHuman trials initiated in July 1998. First patient, first dose in
rheumatoid arthritis December 1998. Clinical studies investigated
patients with rheumatoid arthritis, Crohn’s disease, psoriatic
arthritis, and other diseases, as well as healthy patients.
Important Safety Information
Concurrent administration of CIMZIA with certain biological
DMARDs, including anakinra, abatacept and rituximab, is not
recommended due to an increased risk of serious infections. In
pre-marketing controlled trials of all patient populations
combined, the most common adverse reactions (≥8%) were upper
respiratory infections (18%), rash (9%), and urinary tract
infections (8%).
Please see additional Important
Safety Information continued on the following pages. Full
Prescribing Information can be found here.
is a mobile tool at your patients’ fingertips, helping your patients start and stay on treatment
helps to ensure that your patients get CIMZIA without delaya
for eligible commercial patients through the CIMplicity® Savings Programb
prior authorization support, and specialty pharmacy management
provides CIMZIA education, disease state information, and support servicesc
aCIMplicity®
CoveredTM Eligibility: Eligible
patients with a valid prescription for CIMZIA can receive
treatment with the CIMZIA Prefilled Syringe at no cost for up to
18 months or until the patient’s coverage is approved, whichever
comes first. Program is not available to patients whose
medications are reimbursed in whole or in part by Medicare,
Medicaid, TRICARE, or any other federal or state program or where
otherwise prohibited by law. Patients may be asked to reverify
insurance coverage status during the course of the program. No
purchase necessary. Program is not health insurance, nor is
participation a guarantee of insurance coverage. Limitations may
apply. For initial enrollment into the Program the patient must be
required by his/her commercial insurer to submit a prior
authorization or insurance coverage for the CIMZIA Prefilled
Syringe must be unavailable. To maintain eligibility in the
Program, the following is required: (1) a submitted prior
authorization is denied or coverage remains unavailable for the
patient; and (2) the prescriber must submit an appeal within 45
days of the first two denials and quarterly thereafter. UCB
reserves the right to rescind, revoke, or amend this Program
without notice.
bSavings
Card Eligibility: Available to individuals with commercial
prescription insurance coverage for CIMZIA. Not valid for
prescriptions that are reimbursed, in whole or in part, under
Medicare (including Medicare Part D), Medicaid, similar federal-
or state-funded programs (including any state prescription drug
assistance programs and the Government Health Insurance Plan
available in Puerto Rico), or where otherwise prohibited by law.
Product dispensed pursuant to program rules and federal and state
laws. Claims should not be submitted to any public payor (ie,
Medicare, Medicaid, Medigap, TRICARE, VA, and DoD) for
reimbursement. The maximum annual benefit amount is $15,000 per
calendar year. The parties reserve the right to amend or end this
program at any time without notice. If you are uninsured, other
financial assistance may be available. Call ucbCARES®
toll free at 1-844-599-CARE (2273) for more information. The
CIMplicity® program is provided
as a service of UCB, Inc., and is intended to support the
appropriate use of CIMZIA. Any CIMplicity program may be amended
or canceled at any time without notice. Some program and
eligibility restrictions apply.
cCIMplicity Nurses do not give medical advice and will direct your
patients to share their treatment-related questions with their
clinician.
Important Safety Information
Please see Important Safety Information on the following pages.
Full Prescribing Information can be found
here.
CIMplicity®:
comprehensive support to help start your patients on treatment as soon as possible
Fax a completed enrollment form and the front and back of your patient’s medical and prescription insurance cards to 1-866-949-2469

CIMZIA®
(certolizumab pegol)
is a different
kind of anti-TNF
CIMZIA is the only PEGylated Fc-free anti-TNF8‑10
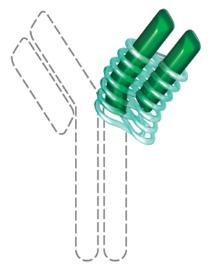
PEGylation can confer beneficial physical and chemical
properties, such as:
Extended half-life of the antigen-binding fragment1,20
Increased bioavailability21,22
CIMZIA is PEGylated: chemically
modified with covalently attached polyethylene glycol8
CIMZIA is a Fab′ fragment
Fab', fragment antigen binding; Fc, fragment crystallizable; IgG1, immunoglobulin G1; IL, interleukin; PEG, polyethylene glycol; TNF, tumor necrosis factor.
Important Safety Information
Concurrent administration of CIMZIA with certain
biological DMARDs, including anakinra, abatacept and rituximab,
is not recommended due to an increased risk of serious
infections. In pre-marketing controlled trials of all patient
populations combined, the most common adverse reactions (≥8%)
were upper respiratory infections (18%), rash (9%), and urinary
tract infections (8%).
Please see additional
Important Safety Information continued on the following pages.
Full Prescribing Information can be found
here.
CIMZIA®
(certolizumab pegol)
Dosing
For patients with psoriasis and psoriatic arthritis, starter dosing of 400 mg (given as 2 subcutaneous injections of 200 mg each) is provided at Week 0 (Day 0), Week 2 (Day 14), and Week 4 (Day 28). This starting dose is the same across all approved indications.1
Maintenance dosing for patients with PSO:
400 mg (2 injections x 200 mg/mL) every
2 weeks
Maintenance dosing for patients with PsA:
200 mg (1 injection x 200 mg/mL) every 2 weeks
— or —
400 mg (2 injections x 200 mg/mL) every 4 weeks


For some PSO patients (with body weight ≤90 kg), a dose of 400 mg
(given as 2 subcutaneous injections of 200 mg each) initially and
at Weeks 2 and 4 followed by 200 mg every other week can be
considered.

OXO, Good Grips® and the associated logos are registered trademarks of Helen of Troy Limited and are used under license.
Important Safety Information
Anaphylaxis or serious allergic reactions may occur. Some of
these reactions occurred after the first administration of CIMZIA.
Hypersensitivity reactions have been reported rarely following
CIMZIA administration. The needle shield inside the removable cap
of the CIMZIA prefilled syringe contains a derivative of natural
rubber latex which may cause an allergic reaction in individuals
sensitive to latex.
Please see additional Important
Safety Information continued on the following pages. Full
Prescribing Information can be found
here.
Study designs
CIMPASI‑1 (Study PS‑1) and CIMPASI‑2 (Study PS‑2)3
Study design was the same for both studies;
CIMPASI‑1, n=234;
CIMPASI‑2, n=227.
Study designs
CIMPASI‑1 (Study PS‑1) and CIMPASI‑2 (Study PS‑2)3
Study design was the same for both studies;
CIMPASI‑1, n=234;
CIMPASI‑2, n=227.
aLoading dose of CIMZIA
400 mg at Weeks 0, 2, and 4 or Weeks 16, 18, and 20.
bOne patient in the CIMZIA 400 mg Q2W group in CIMPASI-2
had prior exposure to ≥3 biologics, which was a protocol
violation.
BIW, twice weekly; ETN, etanercept; LD, loading dose;
PASI, Psoriasis Area and Severity Index; PGA, Physician
Global Assessment; PsA, psoriatic arthritis; Q2W, every
2 weeks; Q4W, every 4 weeks; SJC, Swollen Joint Count;
TJC, Tender Joint Count.
aLoading dose of CIMZIA 400 mg at Weeks 0, 2, and 4 or
Weeks 16, 18, and 20.
bOne patient in the CIMZIA 400 mg Q2W group in CIMPASI-2
had prior exposure to ≥3 biologics, which was a protocol
violation.
Important Safety Information
Please see Important Safety Information on the
following pages. Full Prescribing Information can be found
here.
Study designs
CIMPACT (Study PS‑3)23
Study designs
CIMPACT (Study PS‑3)23
aLoading dose of CIMZIA
400 mg at Weeks 0, 2, and 4 or Weeks 16, 18, and 20.
BIW, twice weekly; ETN, etanercept; LD, loading dose;
PASI, Psoriasis Area and Severity Index; PGA, Physician
Global Assessment; PsA, psoriatic arthritis; Q2W, every
2 weeks; Q4W, every 4 weeks; SJC, Swollen Joint Count;
TJC, Tender Joint Count.
aLoading dose of CIMZIA 400 mg at Weeks0, 2, and 4 or
Weeks 16, 18, and 20.
Important Safety Information
Please see Important Safety Information on the
following pages. Full Prescribing Information can be found
here.
Study designs
The initial, maintenance, and open-label periods of the CIMPASI-1 and CIMPASI-2 phase 3 trials4
Study designs
The initial, maintenance, and open-label periods of the CIMPASI-1 and CIMPASI-2 phase 3 trials4
aDose adjustments were
permitted through Weeks 60–132; dose escalation was
mandatory in patients not achieving PASI 50, and at the
investigator’s discretion in patients achieving PASI 50
but not PASI 75; patients who had received CZP 400 mg Q2W
for at least 12 weeks could have had their dose reduced,
at the investigator’s discretion, if they achieved PASI
75, and were withdrawn if they did not achieve PASI 50.
bPatients entering the open-label period from the CZP 400
mg Q2W escape arm continued to receive CZP 400 mg Q2W but
may have had their dose reduced to CZP 200 mg Q2W at Week
48, at the discretion of the investigator, if they
achieved PASI 75.
CZP, certolizumab pegol; LD, loading dose of CZP 400 mg
Q2W at Weeks 0, 2, and 4, or Weeks 16, 18, and 20; PASI
50/75, ≥50%/75% reduction from baseline in Psoriasis Area
and Severity Index; Q2W, every 2 weeks.
aDose adjustments were permitted through Weeks 60–132;
dose escalation was mandatory in patients not achieving
PASI 50, and at the investigator’s discretion in patients
achieving PASI 50 but not PASI 75; patients who had
received CZP 400 mg Q2W for at least 12 weeks could have
had their dose reduced, at the investigator’s discretion,
if they achieved PASI 75, and were withdrawn if they did
not achieve PASI 50.
bPatients entering the open-label period from the CZP 400
mg Q2W escape arm continued to receive CZP 400 mg Q2W but
may have had their dose reduced to CZP 200 mg Q2W at Week
48, at the discretion of the investigator, if they
achieved PASI 75.
Important Safety Information
Please see Important Safety Information on the
following pages. Full Prescribing Information can be found
here.
Study design
RAPID-PsA (Study PsA001)5
Study design
RAPID-PsA (Study PsA001)5
aLoading dose of CIMZIA
400 mg at Weeks 0, 2, and 4 or Weeks 16, 18, and 20.
bLoading dose of
CIMZIA 400 mg at Weeks 24, 26, and 28.
LD, loading dose; PsA, psoriatic arthritis; SJC, Swollen
Joint Count; TJC, Tender Joint Count; Q2W, every 2 weeks;
Q4W, every 4 weeks.
aLoading dose of CIMZIA 400 mg at Weeks 0, 2, and 4 or
Weeks 16, 18, and 20.
bLoading dose of CIMZIA 400 mg at Weeks 24, 26, and 28.
Important Safety Information
Please see Important Safety Information on the
following pages. Full Prescribing Information can be found
here.
Important Safety Information
Please see Important Safety Information on the following
pages. Full Prescribing Information can be found
here.
Important Safety Information
CIMZIA is contraindicated in patients with a history of
hypersensitivity reaction to certolizumab pegol or to any of
the excipients. Reactions have included angioedema,
anaphylaxis, serum sickness, and urticaria.
Please
see additional Important Safety Information continued on the
following pages. Full Prescribing Information can be found
here.
Important Safety Information You Should Know About CIMZIA® (certolizumab pegol)
Serious and sometimes fatal side effects have been reported with
CIMZIA, including tuberculosis (TB), bacterial sepsis, invasive
fungal infections (such as histoplasmosis), and infections due to
other opportunistic pathogens (such as Legionella or Listeria).
Patients should be closely monitored for the signs and symptoms of
infection during and after treatment with CIMZIA. Lymphoma and
other malignancies, some fatal, have been reported in children and
adolescent patients treated with TNF blockers, of which CIMZIA is
a member. CIMZIA is not indicated for use in pediatric
patients.


