Rapid skin improvement
that can last
CIMZIA 400 mg Q2W demonstrated numerically higher efficacy results for PSO patients than CIMZIA 200 mg Q2W
Individual trial co-primary end points of PASI 75 and PGA 0/1 were statistically significant versus placebo at Week 16 at both doses.3
PASI 100 was a prespecified other end point at Week 16 not adjusted for multiplicity, and a post hoc analysis for Week 16 to Week 144.
*The results of this post hoc analysis should be interpreted with caution as the analysis was not prespecified in the original protocols.
dEstimates presented are
the results from MCMC models where missing data were
imputed using data up to and including Week 48, the end of
the double-blind maintenance period.
eThe co-primary efficacy end points at Week 16 in
CIMPASI-1 and CIMPASI-2 were PASI 75 and PGA 0 or 1.
fThe primary end
point in CIMPACT was PASI 75 at Week 12.
gPGA score of 0 (clear) or 1 (almost clear) based on a
5-point scale (0-4).
MCMC, Markov Chain Monte Carlo; OLE, open-label extension;
PASI, Psoriasis Area and Severity Index; PGA, Physician
Global Assessment; PSO, plaque psoriasis; Q2W, every 2
weeks; TNF, tumor necrosis factor.
aPooled from CIMPASI-1 and CIMPASI-2. Randomized set (Week
0–16); maintenance set (Week 16–48). Missing data were
imputed using multiple imputation based on the MCMC method
using data up to and including Week 48, the end of the
double-blind maintenance period. PASI 100 was a
prespecified other end point at Week 16 not adjusted for
multiplicity, and a post hoc analysis for Week 16 to Week
144. PASI 50 nonresponders at Week 16, 32, or 40 were
imputed as nonresponders at all subsequent time points.
bPlacebo responder rate at Week 16 was 7.5% for PASI 75.
Placebo responder rate was 1.6% at Week 16 for PASI 90.
cPatients taking
CIMZIA 200 mg Q2W received a loading dose of CIMZIA 400 mg
at Weeks 0, 2, and 4.
dEstimates presented are the results from MCMC models
where missing data were imputed using data up to and
including Week 48, the end of the double-blind maintenance
period.
eThe
co-primary efficacy end points at Week 16 in CIMPASI-1 and
CIMPASI-2 were PASI 75 and PGA 0 or 1.
fThe primary end point in CIMPACT was PASI 75 at Week 12.
gPGA score of 0
(clear) or 1 (almost clear) based on a 5-point scale
(0-4).
Important Safety Information
Patients treated with CIMZIA are at an increased risk for
developing serious infections involving various organ
systems and sites that may lead to hospitalization or death.
Patients greater than 65 years of age, patients with
co-morbid conditions, and/or patients taking concomitant
immunosuppressants (e.g. corticosteroids or methotrexate)
may be at a greater risk of infection.
Please
see additional Important Safety Information continued on the
following pages. Full Prescribing Information can be
found here.

CIMZIA®
(certolizumab pegol),
an anti-TNF for your biologic-experienced patients1,2,8-10
Efficacy in Skin That Lasts – Regardless of Prior Biologic Experience2,5
Give your patients the possibility of long‑term efficacy with
CIMZIA®
(certolizumab pegol)
Per protocol, Week 16 placebo nonresponders were initiated on CIMZIA 400 mg Q2W through Week 144.
No protocol-mandated dose reduction in escape population.
Of 116 patients, 33 were dosed down to CIMZIA 200 mg Q2W
at the investigator’s discretion during the Week 16
through Week 144 treatment period.
The results of
the post hoc analyses should be interpreted with caution
as these analyses were not prespecified in the original
protocols.
Patients in the 200 mg Q2W arm continued on 200 mg Q2W out to Week 144
Per protocol, at Week 48 all CIMZIA 400 mg Q2W responders who achieved ≥PASI 50 were dosed down to CIMZIA 200 mg Q2W from CIMPASI-1 and CIMPASI-2. The recommended dose of CIMZIA for adult patients with PSO is 400 mg Q2Wa,c-e
The response in these patients at Week 144 was consistent with the response in patients who continued at 200 mg Q2W to Week 144 in pooled data from the open-label extension period of CIMPASI-1 and CIMPASI-2c-e
Weeks 48 through 144 were an OLE phase of the study. As with any long-term, uncontrolled OLE, there were several limitations with this portion of the study. For example, PASI responder rates did not have a long-term placebo comparator beyond Week 16 (initiation of CIMZIA)
Important Safety Information
Serious and sometimes fatal side effects have been reported
with CIMZIA, including tuberculosis (TB), bacterial sepsis,
invasive fungal infections (such as histoplasmosis), and
infections due to other opportunistic pathogens (such as
Legionella or Listeria). Patients should be closely
monitored for the signs and symptoms of infection during and
after treatment with CIMZIA. Lymphoma and other
malignancies, some fatal, have been reported in children and
adolescent patients treated with TNF blockers, of which
CIMZIA is a member. CIMZIA is not indicated for use in
pediatric patients.
Cases of lymphoma and other malignancies have been observed
among patients receiving TNF blockers, including children,
adolescents, and young adults. Acute and chronic cases of
leukemia have also been reported. Post marketing cases of
hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell
lymphoma that has a very aggressive disease course and is
usually fatal, have been reported in patients treated with
TNF blockers, including CIMZIA. Melanoma and Merkel cell
carcinoma have been reported in patients treated with
TNF-antagonists, including CIMZIA. Periodic skin
examinations are recommended for all patients, particularly
those with risk factors for skin cancer.
Please see additional Important Safety
Information continued on the following pages. Full Prescribing
Information can be found
here.
Post hoc analysis
Pooled PASI responder rates for CIMZIA 400 mg Q2W (n=116) at Week 1442a
No protocol-mandated dose reduction in escape population. Of
116 patients, 33 were dosed down to CIMZIA 200 mg Q2W at the
investigator’s discretion during the Week 16 through Week 144
treatment period.
The results of the post hoc
analyses should be interpreted with caution as these analyses
were not prespecified in the original protocols.
Important Safety Information
Cases of lymphoma and other malignancies have been observed
among patients receiving TNF blockers, including children,
adolescents, and young adults. Acute and chronic cases of
leukemia have also been reported. Post marketing cases of
hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell
lymphoma that has a very aggressive disease course and is
usually fatal, have been reported in patients treated with TNF
blockers, including CIMZIA. Melanoma and Merkel cell carcinoma
have been reported in patients treated with TNF-antagonists,
including CIMZIA. Periodic skin examinations are recommended for
all patients, particularly those with risk factors for skin
cancer.
Please see additional Important Safety
Information continued on the following pages. Full Prescribing
Information can be found
here.
Real patients – real results
Before and after treatment with CIMZIA
Actual clinical trial patients from CIMPACT who reflect CIMZIA use. Individual results may vary.
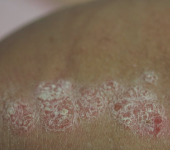
Before - Baseline
Dosing: CIMZIA 400 mg Q2W
PASI score=12
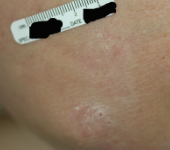
After - Week 16
Dosing: CIMZIA 400 mg Q2W
PASI score=3 |
PASI 75 Achieved
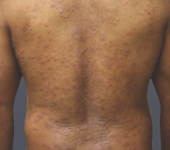
Before - Baseline
Dosing: CIMZIA 200 mg Q2W
PASI score=15
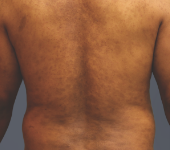
After - Week 12
Dosing: CIMZIA 200 mg Q2W
PASI score=1.8 |
PASI 75 Achieved
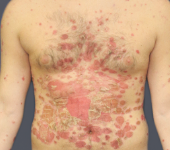
Before - Baseline
Dosing: CIMZIA 400 mg Q2W
PASI score=12.6
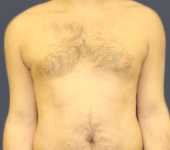
After - Week 48
Dosing: CIMZIA 400 mg Q2W
PASI score=0 |
PASI 100 Achieved
BIW, twice weekly; PASI, Psoriasis Area and Severity Index; PSO, plaque psoriasis; Q2W, every 2 weeks. For patients who were originally assigned to CIMZIA 400 mg Q2W and maintained this dosing through Week 48 (N=49), the PASI 100 responder rate was 45% (NRI).
Important Safety Information
In pre-marketing controlled trials of all patient
populations combined, the most common adverse reactions (≥8%)
were upper respiratory infections (18%), rash (9%) and urinary
tract infections (8%).
Rare reports of blood
dyscrasias have been reported with CIMZIA; patients should be
advised to seek medical attention if they develop. Patients on
CIMZIA should not receive live or live-attenuated vaccines.
Concurrent administration of CIMZIA with certain biological
DMARDs, including anakinra, abatacept and rituximab, is not
recommended due to an increased risk of serious infections.
Please
see additional Important Safety Information continued on the
following pages. Full Prescribing Information can be found here.
Proven efficacy regardless of prior biologic treatment
Comparable skin clearance in biologic-experienced vs biologic-naïve patients
*The results of the biologic-experienced PASI 90 data
were part of the post hoc analysis and should be
interpreted with caution as the analysis was not
prespecified in the original protocols.
Subjects
must not have been exposed to more than 2 biological
response modifiers (including anti-TNF) for PsA or PSO
prior to Baseline Visit.
aPrimary biologic
nonresponse was an exclusion criterion in all 3 trials.
Missing data were imputed using multiple imputation based
on the MCMC method. The PASI 75 analysis is a prespecified
pooled efficacy analysis of the CIMPASI-1, CIMPASI-2, and
CIMPACT trials. The results of the biologic-experienced
PASI 90 data were prespecified in the integrated summary
of efficacy analysis but not adjusted for multiplicity.
bPatients taking CIMZIA 200 mg Q2W received a loading dose
of CIMZIA 400 mg at Weeks 0, 2, and 4.
IL, interleukin; MCMC, Markov Chain Monte Carlo; PASI,
Psoriasis Area and Severity Index; PSO, plaque psoriasis;
Q2W, every 2 weeks; TNF, tumor necrosis factor.
aPrimary biologic nonresponse was an exclusion criterion
in all 3 trials. Missing data were imputed using multiple
imputation based on the MCMC method. The PASI 75 analysis
is a prespecified pooled efficacy analysis of the
CIMPASI-1, CIMPASI-2, and CIMPACT trials. The results of
the biologic-experienced PASI 90 data were prespecified in
the integrated summary of efficacy analysis but not
adjusted for multiplicity.
bPatients taking CIMZIA 200 mg Q2W received a loading dose
of CIMZIA 400 mg at Weeks 0, 2, and 4.
Important Safety Information
Use of TNF blockers, including CIMZIA, has been
associated with reactivation of hepatitis B virus in
patients who are chronic carriers of the virus. In some
instances, HBV reactivation occurring in conjunction with
TNF-blocker therapy has been fatal.
Cases of
worsening congestive heart failure (CHF) and new onset CHF
have been reported with TNF blockers. Treatment with TNF
blockers, including CIMZIA, may rarely result in new onset
or exacerbation of clinical symptoms and/or radiographic
evidence of central or peripheral nervous system
demyelinating disease, as well as the development of a
lupus-like syndrome.
Please see additional
Important Safety Information continued on the following
pages. Full Prescribing Information can be found here.
Proven efficacy regardless
of prior
biologic treatment
Of the 850 patients randomized to receive placebo or CIMZIA in
these studies, 30% had received prior biologic therapy* for the
treatment of psoriasis (14% with ≥1 anti-TNF agent and 16% with an
anti-IL agent)1
*Reason for
discontinuation of prior biologic treatment unknown.
The CIMplicity® Navigator™ provides patient services designed for ease of patient initiation.
Please see Important Safety Information on the following pages.
Full Prescribing Information can be found here.
The CIMplicity® Navigator™ provides patient services designed for ease of patient initiation.
Please see Important Safety Information on the following pages. Full
Prescribing Information can be found
here.







